از دکتر بپرسید!
در این بخش میتوانید سوالات خود در رابطه با بیماریهای ریوی را مطرح نمایید و دکتر در اسرع وقت پاسخگوی سوالات شما خواهد بود.
رزرو وقت آنلاین
در این بخش شما کاربران عزیز میتوانید قرار ملاقات خود با دکتر را رزرو کنید و هزینه را به صورت آنلاین پرداخت نمایید.
بیماریهای ریه
در این قسمت از وبسایت مطالب مفیدی در رابطه با بیماریهای ریه گنجانده شده است. ضمن اینکه نظرات کاربران هم زیر هر مطلب موجود است.

دکتر علیرضا ثابت پور
فوق تخصص ریه و متخصص داخلی
دکتر علیرضا ثابتپور از بهترین پزشکان حال حاضر کشور در زمینه بیماریهای ریه هستند. دکتر ثابت پور فوق تخصص ریه و متخصص داخلی هستند. تمام دورههای رزیدنتی و فلوشیپی ایشان آموزشی-تحصیلی بوده و زیرنظر و مورد تائید رسمی ریال کالج انگلستان قرار گرفته است. مراحل سه گانه امتحان عضویت در کالج سلطنتی متخصصان داخلی هم با موفقیت گذارندهاند که معادل امتحانات بورد ایران است.
بیمارستان پپورث کمبریج( Papworth hospital, Cambridge) زیر نظر استاد دکتر پارمار
- آشنایی با بیماریها و علل منتهی به پیوند ریه
- عوارض عفونی و غیرعفونی پیوند ریه
- علل رد پیوند ریه
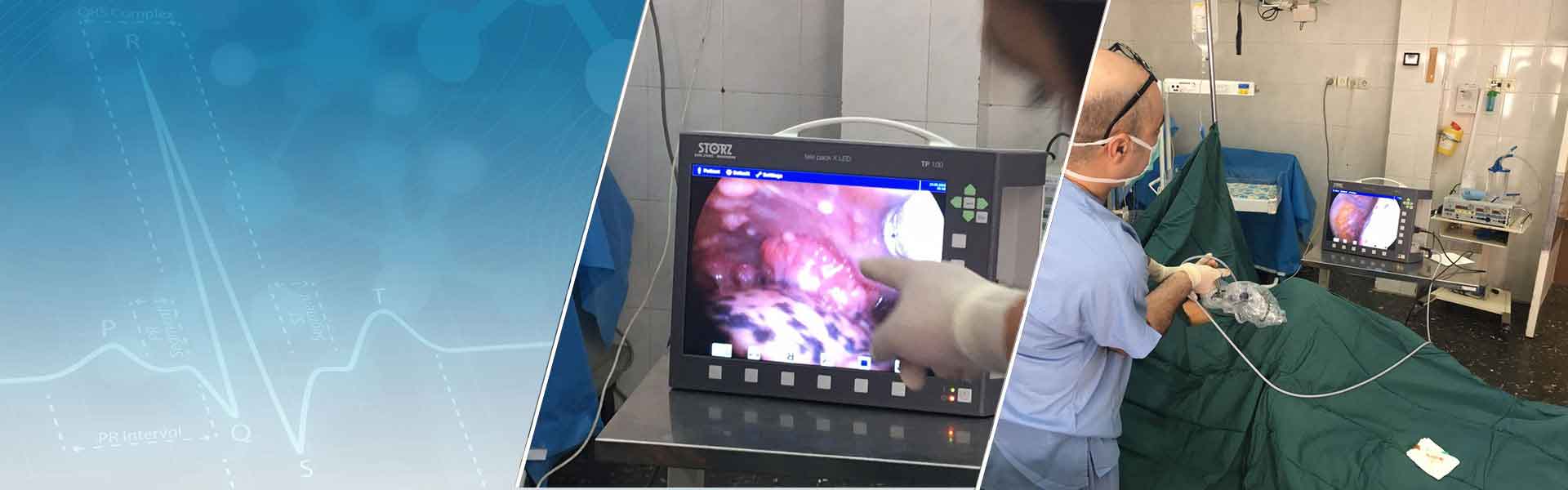
- برونکوسکوپی بر روی ریه پیوندی
- برونکوسکوپی با تکنیک الکتروکوتری؛ باز کردن مجاری تنگ با لالون و قرار دادن استنت
- بیماریهای داخلی؛ زیر نظر دکتر والش (Hinchinbrookehospital, Cambridge)
- بیماریهای داخلی کهنسالان؛ زیر نظر دکترآمار( Royal Bournemouth hospital)
- بیماریهای گوارش زیر نظر دکتر تلی( Royal Lincholnshire hospital)
- بیماریهای داخلی کلیه
- بیماریهای قلب و ریه زیر نظر پروفسور پیورد و بنت ( Leicester and Glenfileld hospital)
بیمارستان پپورث کمبریج ( Papworth hospital, Cambridge) زیر نظر اساطید دکتر رابرت رینتول و مارک اسلید.
- آشنائی با درمان و شیوههای نوین شناخت سرطان ریه
- چگونگی برخورد تیمی بجای تصمیم فردی با بیمار سرطان ریه
- آشنایی و انجام برونکوسکوپی با تکنیک سونوگرافی ( Endobronchial (Ultrasound, EBUS جهت تشخیص و مرحلهبندی سرطان ریه
- آشنایی با تکنیک برونکوسکوپی با تکنیک کرایو جهت درمان بیماران سرطانی
بیمارستان لنکستر؛ منچستر( ( Royal Lancaster Infirmary زیر نظر اساتید دکتر ویلی و اندریانی
- آشنایی با بیماریهای ریه
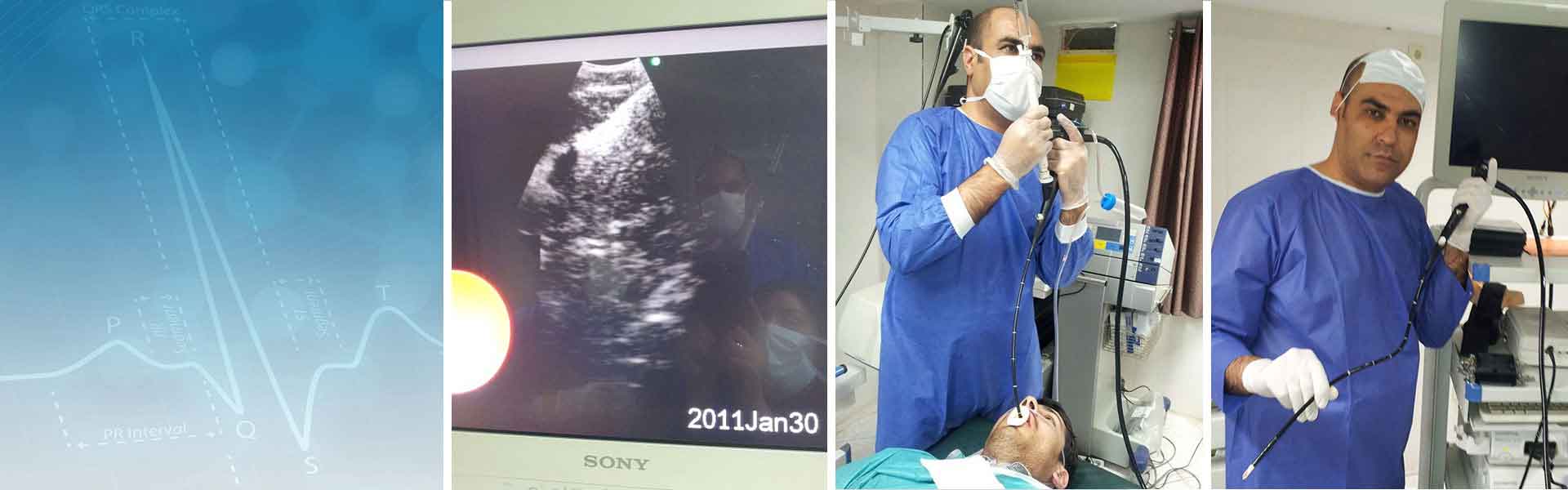
- انجام برنکوسکوپی
- بیوپسی پرده پلورال و سونوگرافی ریه
- انجام چست تیوب و تخلیه مایع پلورال
- آشنائی با مکانیسم تهویه با فشار مثبت و مراقبتهای ویژه
ایمنوپاتولژی آسم
ایمنولوژی آسم: گرچه به طور کلاسیک آسم با التهاب راه های هوایی که توسط IL4 , 5 , 13 یا در واقع سایتوکاین های ILC2 هستند شناخته می شوند و باعث می شوند میزان ائوزینوفیل ها حساسیت های ریوی و ترشح IGE...
تازه های فیبروز ریوی
پاتوژنسیس و درمان IPF : فیبروز ریه یک بیماری پیشرونده است و در نهایت منجر به نارسایی تنفسی با افزایش ECMD می شود. شروع فیبروز با یک تحریک متوالی شروع می شود و منجر به ترمیم زخمی می شود که...
ARDS و کرونا
ایمنوپاتولوژی ARDS : این بیماری باعث مرگ و میر زیادی به علت تجمع مایع پروتئینی در آلوئول ها می شود. شایع ترین علت آن ویروس ها و باکتری ها هستند و به ندرت قارچ. در شرایط دیگری مانند سپسیس سوختگی...
نقش احتمالی پرفنیدون در کرونا
اثر پرفنیدون در کرونا : پرفنیدون باعث کاهش تی ان اف آلفا و بسیاری دیگر از سایتوکاین های التهابی می شود. پرفنیدون همچنین NLP – P3 را که موجب التهاب می شوند را مهار می کنند. پرفنیدون به شدت TGF/β1 که...
ضایغات لنفوراتیو ریه
که این بیماری را بدون لینفوماتوئیدگرنولوماتوزیز: این بیماری یک بیماری لنفوم ناشایع همراه با عفونت EBV می باشد. معمولا در بیماران با ایمنی با نقص ایمنی یا بیماران با اتوایمون – ایدز یا در بیماران...
اینترلوکین 1 یا 6
مهار اینترلوکین ۱ همراه است با کاهش مرگ و میر در بیماران کرونایی بستری در بیمارستان. مهار اینترلوکین ۶ اما در گروهی که CRP بالای دارند موثر می باشد. مهار هر دو در بیماران با LDHپایین موثر است....
تازه ترین پرسشهای کاربران
بهترین متخصص ریه در تهران
متخصص ریه خوب در تهران
بهترین فوق تخصص ریه در تهران
دکتر فوق تخصص ریه در تهران
دکتر فوق تخصص عفونت ریه
فوق تخصص سرطان ریه